ACKNOWLEDGEMENT:
Figure
|
Source of Figure
|
Figure 14
|
https://sites.google.com/site/internationalgcsechemistry/
|
Preparation of
Ethanol:
Ethanol is produced by 2 processes.
- Fermentation of glucose
- Addition of steam to ethene
Fermentation of
glucose:
Glucose decomposes to form ethanol and carbon dioxide, in the
absence of oxygen and presence of yeast as a catalyst.
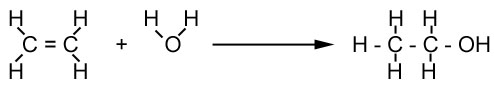
Addition of steam to
ethene:
Ethene reacts with steam, at 300°C temperature and 60
atmosphere pressure, with Phosphoric acid as a catalyst, to form ethanol.
Figure 14:
This process is used during the industrial manufacture of
Ethanol.
Uses of Ethanol:
- Ethanol is used as a solvent in:
- Sanitizers
- Cosmetics
- Antitussive
- Glues
- Medicines
- Ethanol is used as a renewable source of energy.
- Ethanol is used as a major constituent of alcoholic beverages.