ACKNOWLEDGEMENT:
Figure
|
Source of Figure
|
Figure 5a
|
http://www.docbrown.info
|
Figure 5b
|
http://brainly.in
|
Figure 5c
|
www.meritnation.com
|
Electrolysis of Copper (II) Sulphate solution:
The products
of the electrolysis of Copper (II) Sulphate solution depends on the kind of
electrodes used during electrolysis.
i.
Using inert (graphite) electrodes:
Figure 5a:
Ions present in the
solution = Cu2+(aq) + SO4-2-(aq)
+ H+(aq)+ OH-(aq)
Ions at the anode:
SO4-2 and
OH-
Reaction at the anode:
Ions at the cathode:
Cu+2 and H+
Reaction at the cathode:
The blue colour of the
solution will gradually fade as copper is discharged and deposited on the
cathode.
ii.
Using reactive electrodes:
Figure 5b:
When reactive electrodes
are used the anode dissolves.
Ions present in the
solution = Cu2+(aq) + SO4-2(aq)
+ H+(aq)+ OH-(aq)
Ions at the anode:
SO4-2 and
OH-
Reaction at the anode:
Anode dissolves:
The dissolving anode also deposits impurities in the electrolytic cell. This is called anode mud.
Ions at the cathode:
Cu+2 and H+
Reaction at the cathode:
Copper being easier to
discharge than hydrogen will be obtained at the cathode, in both cases. It will
settle on the cathode, making it heavier by increasing its mass. The blue
colour of the solution remains the same; as the copper ions discharged at the cathode
are continuously being replaced by copper ions from the dissolving anode, to
maintain the concentration of the solution. The reaction continues till all the
anode immersed in the solution is dissolved.
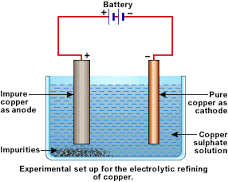
This
technique is typically used during the refining of copper. Impure copper is
made the anode while a 99.99% pure copper wire is used as the cathode.
Figure 5c:
The impure copper anode dissolves and the copper ions travel to the cathode. Impurities like sand in the impure anode are non-conductors and settle at the bottom of the cell as anode sludge. Copper is refined using this process. The 99.99% pure copper cathode is used to make wires for electricity transmission and distribution.