ACKNOWLEDGEMENT:
Figure
|
Source of Figure
|
Figure 8a
|
https://prezi.com/myiigflss0gg/charless-law/
|
Figure 8b
|
https://prezi.com/myiigflss0gg/charless-law/
|
Figure 8c
|
www.physics-reference.com
|
Gases & the effect of temperature and pressure on their volumes:
The particles of the gas are far
apart, moving in random directions with their K.E. Therefore, there are large
empty spaces between the particles which gives the gases their compressibility.
For a fixed volume of gas in a gas jar, the volume of a gas is affected by
temperature and pressure.
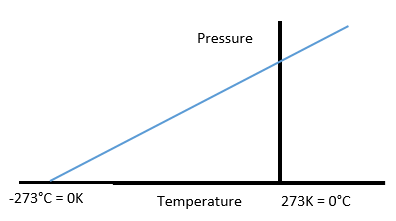
Temperature in Celsius = Temperature in Kelvin – 273
Temperature – Volume Graph:
Absolute Temperature:
Absolute temperature is -273°C or 0
Kelvin; K. It is theoretically the lowest and coldest temperature possible and
0 Kelvin is called as Absolute Zero. At this temperature the particles of
matter have minimal or no energy and motion.
The size of the Kelvin and Celsius scale
is same so all temperatures in Celsius can be converted to their Kelvin values
by adding 273. All Kelvin temperatures can be converted to Celsius by
subtracting 273.
Temperature in Kelvin = Temperature in
Celsius + 273
Temperature in Celsius = Temperature in Kelvin – 273
0°C = 273K
-273°C = 0K
Temperature – Volume Graph:
Temperature – Pressure Graph:
Case 1: Effect of temperature on Volume of a gas, at Constant Pressure:
Figure 8a:
 |
| Figure 8b: |
Charles’ Law stated this effect
as:
“The Volume of a gas is directly
proportional to its absolute Temperature, at Constant Pressure.”
Mathematically:
Graphically: Figure 8c