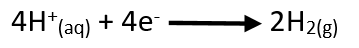ACKNOWLEDGEMENT:
Figure
|
Source of Figure
|
Figure 3
|
http://www.dynamicscience.com.au |
Hydrogen:
Manufacture of Hydrogen:
Hydrogen can
be prepared by the following reactions.
- Electrolysis of water
- Cracking of Hydrocarbons usually alkanes
Electrolysis of water:
Pure
distilled water is a non-conductor of electricity. A few drops of concentrated
sulphuric acid are added to water to make it an electrolyte. These drops of
sulphuric acid decomposes water molecules into the ions of H+ and OH-.
Figure 3 shows the apparatus used for this reaction.
Figure 3:
Negative ions present in the
electrolyte:
SO4-2(aq)
and OH-(aq)
Reaction at the anode:
Positive ion present in the
electrolyte:
H+(aq)
Reaction at the cathode:
Overall, reaction of
the cell:

Cracking of Hydorcarbons:
Hydrogen can
be obtained from the cracking of Hydrocarbons.
Hydrogen as a fuel:
Hydrogen can
be used as a fuel of the future as it reacts with oxygen to form water.
Advantages:
- Non-polluting product, water is formed
- Evolve large amounts of heat
Disadvantage:
- Dangerous
- Large amount of heat can lead to accidents