Presence of Carbon and Hydrogen in
the reactivity series:
Two
non-metals Carbon and Hydrogen are present in the reactivity series. This is
because Carbon and Hydrogen can, like more reactive metals, reduce metals below
them in the series.
Example:
This
reducing action of Carbon is utilized during the extraction of iron from its
ore.
Example:
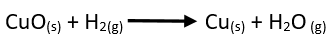
Hydrogen gas
is passed over heated Copper oxide in a vacuumed tube. Hydrogen reduces Copper
oxide to copper and forms water as a by-product.
Metal carbonates and their stability
to heat:
All metal
carbonates decompose to form metal oxides and carbon dioxide when heated. The
amount of heat required for thermal decomposition suggest the stability of the
carbonate. More reactive metals like Sodium and Potassium form more stable
carbonates with high decomposition temperatures. As we move down the series, the
decomposition temperatures decreases with calcium carbonate decomposing at
around 850°C and magnesium carbonate at 350°C, while copper carbonate is
decomposed to copper oxide and carbon dioxide in a lab using a Bunsen burner.
Thus, stability of carbonates decreases down the series.
Table 4:
Metals at
the bottom of the series prefer to stay in atomic form.
Practical Example: Gold and Platinum are found uncombined in the earth's crust.