ACKNOWLEDGEMENT:
Figure
|
Source of Figure
|
Figure 19
|
http://www.bbc.co.uk
|
Figure 20
|
study.com
|
Figure Table 8a
|
http://commons.wikimedia.org/
|
Figure Table 8b
|
http://commons.wikimedia.org/
|
Figure Table 8c
|
http://janison.cyriljackson.wa.edu.au/
|
Figure Table 8d
|
http://www.ivyroses.com/
|
Figure Table 8e
|
-
|
Figure Table 8f
|
http://imgarcade.com/
|
Figure Table 8g
|
http://www.gcsescience.com/
|
Figure Table 8h
|
http://www.ivyroses.com/
|
Esters:
Esters are formed as a product of the reaction between
alcohols and carboxylic acids, in the presence of concentrated sulphuric acid
as a catalyst. Water is released as a by-product of this reaction.
Figure 19 shows the reaction:
Figure 19:
The H radical from the alcohol and the Hydroxyl; -OH radical
from the acid combine to form water.
The ester is bonded by the ester linkage which is bonded by
the ester linkage.
Figure 20:
Since, 2 reactants condense to form a single compound and
release water as a by-product, this is also known as Condensation Polymerization.
Naming convention of
Esters:
Consider the following table.
Table 8:
Ester
|
Chemical Formula
|
Structure
|
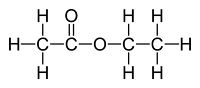
Methyl Methanoate
|
HCOOCH3
|
|
Methyl Ethanoate
|
CH3COOCH3
|
|
Methyl Propanoate
|
C2H5COOCH3
|
|
Methyl Butanoate
|
C3H7COOCH3
|
|
Ethyl Methanoate
|
HCOOC2H5
|
|
Ethyl Ethanoate
|
CH3COOC2H5
|
|
Ethyl Propanoate
|
C2H5COOC2H5
|
|
Ethyl Butanoate
|
C3H7COOC2H5
|
Note the following 2
points:
- The structure read from left to right shows that the acidic portion of the ester first, followed by the – COO – bond, and then the alcohol portion.
- In the naming convention, the alcohol name comes first, followed by the name of the acid used, ending with –ate.
Esters are sweet smelling compounds. They, thus have a large
variety of industrial uses such as:
- Artificial flavourings in food industry
- Scents in perfume industry
- As a solvent