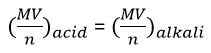ACKNOWLEDGEMENT:
Figure
|
Source of Figure
|
Figure 3
|
www.rsc.org
|
Preparation of Salts:
Salts can be
prepared by the reaction between acids and metals, bases and carbonates. (as
explained in the properties of Acids). The techniques used are as follows:
- Titration
- Precipitation
Titration:
In
titration; an acid of a known or unknown concentration is added from a burette to a base
of known or unknown concentration in a flask. The acid is added till the required colour
change is obtained using an indicator. The change of the colour of the indicator marks the end of the neutralization reaction. This method is used to prepare
soluble salts.
Figure 3:
Soluble
salts prepared by this method are obtained from their solutions, by heating the
solution to evaporate excess water from the solution and then crystallization
the solution to form salt crystals.
Mathematics of Titration:
The equation
of titration is given by:
Let’s consider the following equation for explaining the equation of titration:
M =
Concentration of acid/alkali in moles/dm3
V = Volume
of acid/alkali used in cm3
n = number
of moles used according to the balanced chemical equation
Example:
here, nacid = 1 but nalkali = 2, because 1 mole of the acid requires 2 moles of the alkali.