ACKNOWLEDGEMENT:
|
Figure
|
Source of Figure
|
|
Figure 5a
|
www.bbc.co.uk
|
|
Figure 5b
|
socratic.org
|
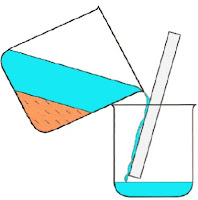
Precipitation:
Precipitation
is the method used to prepare insoluble salts. In this method 2 soluble
solutions are reacted together to yield an insoluble salt. The insoluble salt is obtained by using filtering the solution and washing the residue insoluble salt with
water and drying it, or decanting as shown in Figure 5a and 5b, respectively.
Figure 5a:
Figure 5b:
Example: Barium sulphate is prepared using
aqueous solutions of barium chloride and sodium sulphate.
Salts and
their solubilities:
Table 5: Salt and their solubilities
are shown in the Table below.
|
Soluble
|
Insoluble
|
|
All nitrates
|
None
|
|
All Chlorides except
|
Silver chloride; AgCl
Lead chloride PbCl2
Mercury chloride HgCl2
|
|
All Sulphates except
|
Cacium sulphate; CaSO4
Barium sulphate; BaSO4
Lead sulpahte; PbSO4
|
|
Sodium carbonate; Na2CO3
Potassium carbonate; K2CO3
Calcium carbonate; CaCO3
|
All Carbonates except
|