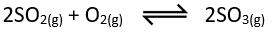Sulphuric Acid:
Sulphuric
acid is an extremely important industrial product. Many industries depend on
using sulphuric acid.
Industrial manufacture of Sulphuric
Acid:
Sulphuric
Acid is industrially manufactured in a 7 step process, listed below:
- Sulphur from the earth’s crust is burned in the presence of Oxygen to form Sulphur dioxide.
- Sulphur dioxide is purified by passing through electrostatic dust precipitators to remove arsenic impurities. Arsenic impurities decrease the efficiency of Vanadium(V) oxide catalyst.
- Sulphur dioxide and Oxygen are washed with water and dried before reaction.
- Sulphur dioxide is reacted with more Oxygen to form Sulphur trioxide. This is a reversible reaction and therefore, the gaseous mixture is reacted at a temperature of 450°C and 2 to 3atm pressure, in a chamber of heated Vanadium(V) oxide mesh as a catalyst. This is the most critical step of the process and this manufacture of Sulphur trioxide is also known as the Contact’s Process.
- The conditions for this process are strictly monitored and maintained to ensure a maximum yield of Sulphur trioxide.
- Sulphur trioxide obtained by Contact’s process is dissolved in Sulphuric acid to form a dense liquid called, Oleum.
- This
oleum is diluted with distilled water to form Sulphuric acid.
Uses of Sulphur dioxide:
- As a bleaching agent
- In the manufacture of wood pulp for paper
- As an antibacterial for food preservatives
Uses of Sulphuric acid:
- Detergents
- Fertilizers
- Battery acid