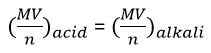Nitrogen and Ammonia:
Ammonia is
an industrially important gas and is widely used to manufacture fertilizers. It
is industrially manufactures using Hydrogen and Nitrogen by the Harber’s
Process.
Harber’s Process:
Nitrogen,
obtained from the fractional distillation of liquid air and Hydrogen from
Cracking of crude oil are made to react together in volumes of 1 : 3,
respectively, at 450°C and 300atm in presence of Iron as a catalyst to yield Ammonia.
This is a
reversible reaction; therefore the conditions are strictly maintained to ensure
the maximum yield of Ammonia. It is an exothermic reaction; giving out heat and prefers low temperatures. The rate of the reaction however, decreases at low temperatures, therefore the temperature of 450°C is preferred to ensure a fast and maximum yield of ammonia,